\( \require{amstext} \require{amsmath} \require{amssymb} \require{amsfonts} \)
Definition/Conceptual of Medical Devices
Biocompatibility
- In terms of bio-safety: The medical device does not cause harm to organism, the device has no cytotoxicity, carcinogenicity
- In terms of bio-functionality: The material performs required host response in a specific use.
Bioethics:
- Respect for person: The choices of an autonomous individual should be respected, and those individuals could not make decisions should be protected.
- Beneficence: The participation of a research study should have a potential beneficial balance of potential harm and potential benefits
- Justice: The researchers should not exploit vulnerable individual or leave out anyone who might be beneficial from the study without good reason.
Medical Devices Classification
The biomedical devices are classified by the potential risks and the vulnerability of the human body to the device. The higher the risks, the higher the classification. In EU, there are 5 classes: I-non sterile, I-sterile, IIA IIB, III. In US, there are 3 classes: I II III.
Sterilization
Consideration of sterilization:
- Scale of manufacturing. This relates to the economics of using larger capitaled devices.
- The required mechanical or chemical properties. This might be altered by any chemicals, heat (melts), EM radiations (plastic).
- Geometry. Heat specifically
- The packaging during the sterilization, and the bulk size of the sterilization
- The residue of the sterilization, will it be toxic and cause biocompatibilty
- How to measure and validate the process should be easy and clear.
- The sterilisation speed, does it require qurantining of the product.
- The cost of the operation.
Examples of sterilization techniques:
- Steam:
- High temperature
- The material absorbed the moisture
- Low Cost
- Ease of control, fast, no toxic residue
- Gamma Radiation:
- High capital overlay
- Radioactive isotopes
- Plastic degradation
- Can penetrate packages easily
- No residue
- Ethylene oxide EO:
- Toxic residue, need to quarantine for 1-2 weeks
- Many process variable to control
- Good efficacy, good compatibility with different materials
- Could be sterilized after packaged Less established: E-beam, X-ray, Pulsed Light, Hydrogen peroxide gas plasma
Validation of the sterilization product:
- The validation can be done with the process control rather than the final product.
- Test the most resistant organism for a certain type of sterilization technique.
- Bioburden Count the microorganisms before and count after sterilization for a period of time and interpolate to the desired SAL (sterilization assurance level)
- SAL usually set to $10^-6$ 1 in a million chance that the product is not sterilized.
Polymer
Polymer Characteristics
- Degree of polymerization: n the number of monomers in the polymer molecules
- Since polymers are synthesized from monomers, not all polymer chains will grow to same length. Molecular weight has a such distribution profile.
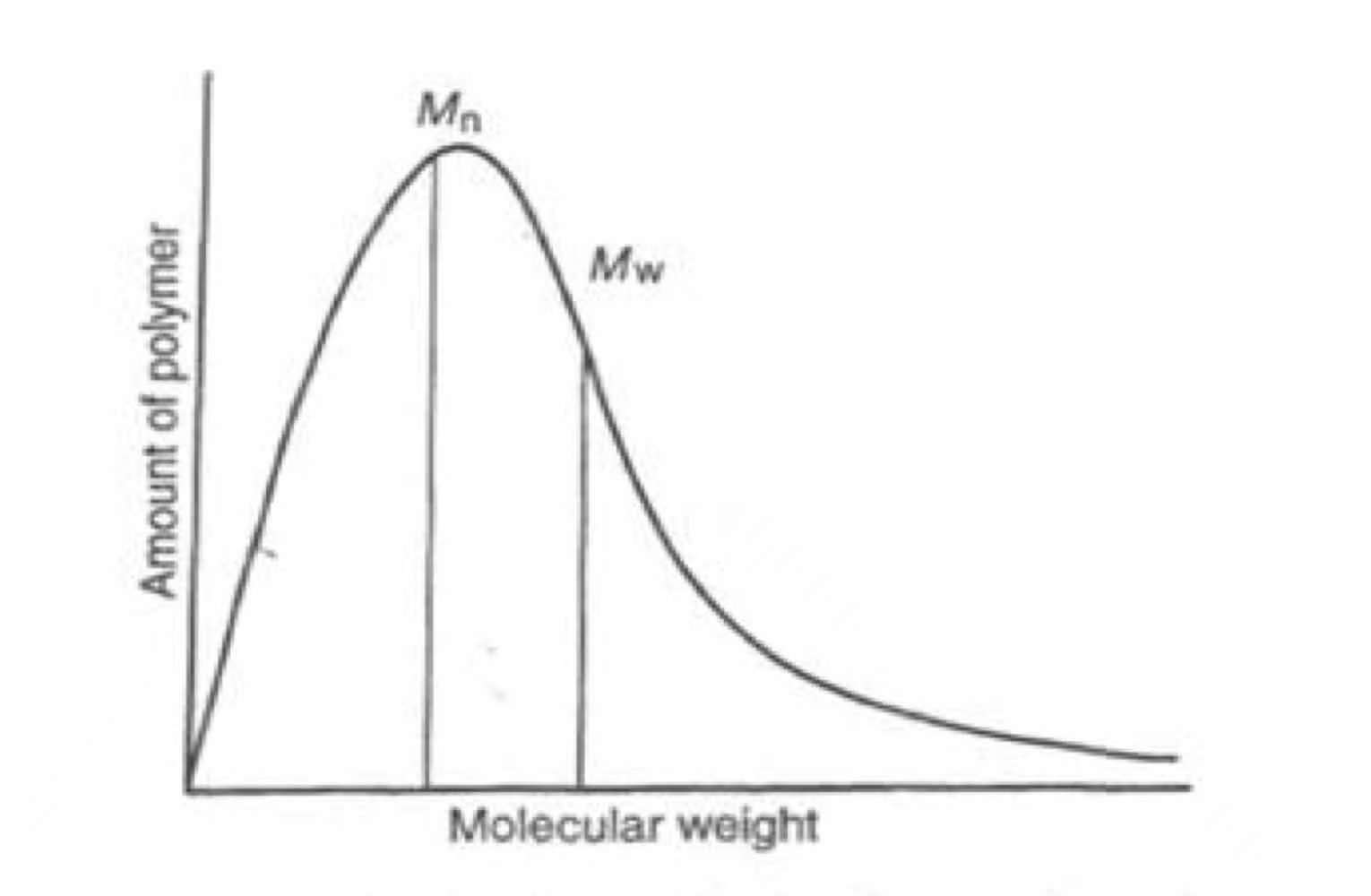
- Molecular weight Polydispersity index: \( \textrm{PDI} = \frac{M_w}{M_n} \) where $M_w$ is the weight average molecular weight, $M_n$ is the number average molecular weight.
Mechanical Properties of polymers
-
Melting point: Longer the chain length (n), the higher the melting point. Since more thermal energy is required to overcome the inter-chain van der Waals forces.
-
Crystallinity: Higher cross-linked structure, lower likely to be crystallinity. It requires ordered chain packing. Polymers with narrow distribution of molecular weight (PDI closer to 1) will have more similar chain length and more possible to have ordered chain packaging.
-
Young’s Modulus: The longer the chain the higher the moduli. It varies approximately linearly with n before n reaches $10^5$.
Examples of Application of Biocompatible Polymers
- PTFE for vascular grafts:
- Requires good strength, toughness, fatigue resistance in the ~ 10 years life of the grafts for the blood pressure.
- Requires the surface to be smooth, inert, non-adhesive and wear resistance to the blood flow and should be biocompatible to the blood cell.
- The increase in crystallinity of the polymer would improve the required mechanical properties
- PGA 90% / PLA 10% Co-polymer for resorbable sutures
- Biodegradable. Half life of 3 weeks, it is designed and tuned to be suited to the time the wound heal after a surgery
- The bio-degraded by product of this material is non-toxic and could be reabsorbed by the body
- Gives higher crystallinity compared to PLA rich polymer and have better mechanical properties for it to be suture application
- Polyanhydrides made into wafers for brain tumor drug delivery
- Biodegradable. Half life of several days, designed and tuned to fit the period of drug release.
- The half-life allowed the drug carried by the polymer to controlled released in the brain tumor area with time scale of several days
- The controlled release properties are more important than its mechanical properties, an amorphous material is used here.
- Collagen fibre scaffold as haemostatic dressing
- The crystalline fibre arises from the quaternary structure of collagen I.
- When Platelets meet the collagen fibre, clots form to stop bleeding.
- Melting of banded structure prevents clotting and down-regulate the inflammatory response.
- PGA 50% PLA 50% for drug delivery/
Drug Delivery
Drug release
- Drug along suffers from low solubility, unstable, fast clearance out of body, short circulation time, non-specific toxicity.
- Three main function provided by drug release:
- Controlled release
- The drug would be more effective if the concentration in the body or an organ are controlled at a certain level during the treatment window. This could be achieved with a drug carrier with specifically designed polymer that have wanted diffusion, dissolution and degradation rate in both temporal and spatial.
- Targeted release
- Some toxic drug might be best delivered to the certain area of body before release, say brain tumor to avoid increasing toxicity and increase the efficacy of the drug release. This could be achieved with coating or conjugating the drug with affinity reagents such as nucleic acid, antibodies, polysaccharides
- Enhanced solubility
- Some drug might be less effective if not stable, or low in solubility, this could be solved with conjugating the drug with some polymers or encapsulated in drug delivery carrier.
- Controlled release
- Co-polymer are mainly used for controlled release. The co-polymer is an amorphous form such that the polymer carrier can dissolve and degrade homogenously and therefore the drug could release uniformly. The release rate can also be controlled in amorphous form.
Hydrolysis:
- Hydrolysis is the process of the breakdown the polymer chain covalent bond when the polymer contacts with water.
- Hydrolysis rate depends on:
- Most importantly, the material property, the polymer backbone
- The hydrophilic or hydrophobic nature of the repeating group in the polymer
- The geometry, say the surface area to volume ratio
- Polymer crystallinity
- Polymer rubbery or glassy state rubbery faster
- Porosity
Bulk vs Surface erosion
- Diffusion time scale:
\(
\tau_D = \frac{\pi
^2}{4D_{eff}} \\) - Hydrolysable bond degradable time scale:
\(
\tau_E = 1/\lambda (\ln(
) - \ln((V)^{1/3})) \\) - $\tau_D < \tau_E$ Bulk erosion
- $\tau_D > \tau_E$ Surface erosion